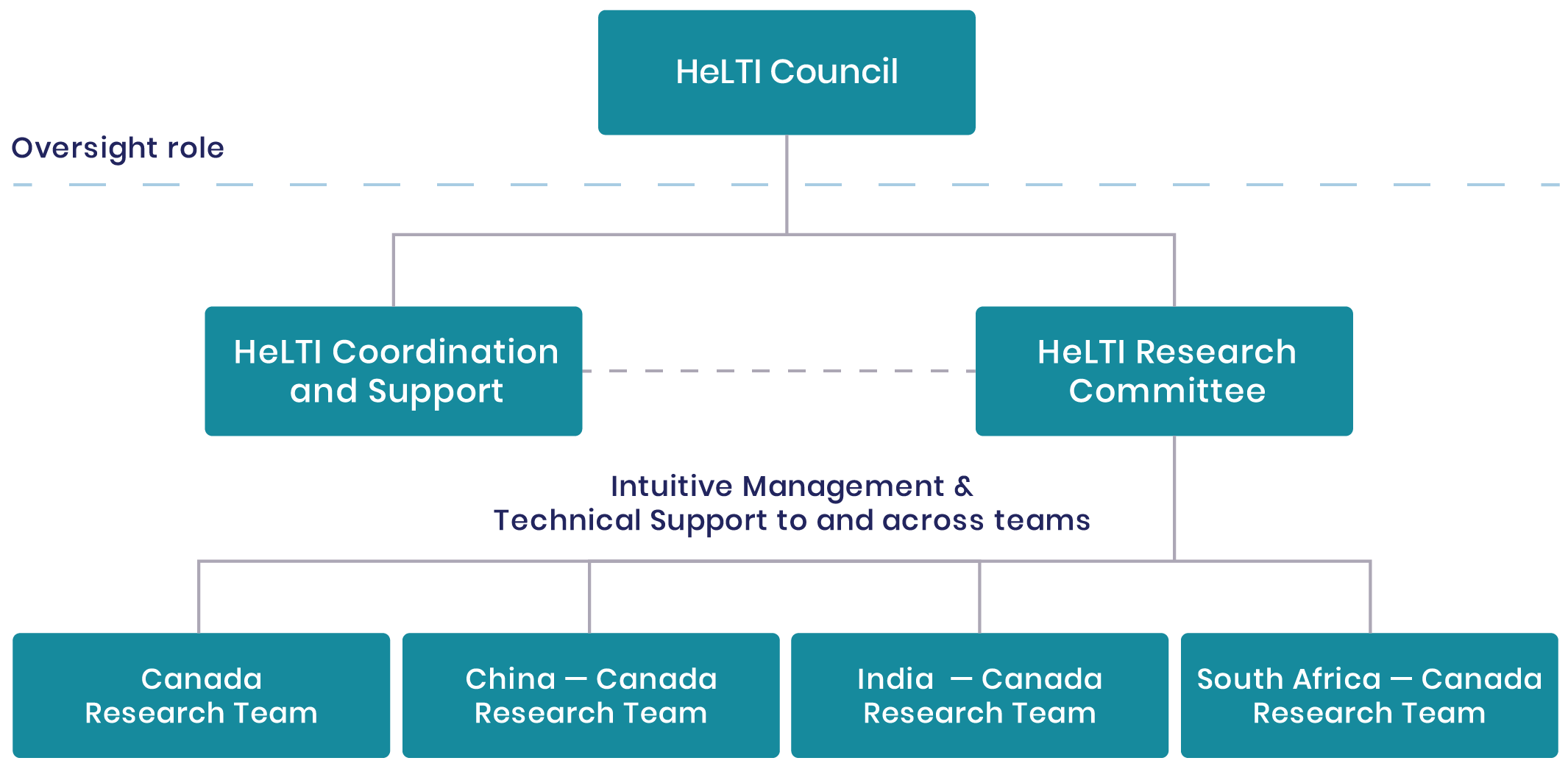
HeLTI Council
The HeLTI Council enables the success of the HeLTI linked international intervention cohorts through oversight and strategic leadership of the initiative. Membership includes two individuals from each participating funding body – (Canada: Canadian Institutes of Health Research (CIHR); China: National Natural Science Foundation of China (NSFC); India: Department of Biotechnology (DBT); and South Africa: South African Medical Research Council (SAMRC)), and World Health Organization (WHO). The Chair rotates annually between country members.
HeLTI Research Committee
The HeLTI Research Committee (RC) is responsible for promoting scientific collaboration and harmonization across the HeLTI teams. Membership includes two principal investigators from each joint team to include the Canadian and international lead principal investigators. The WHO Secretariat also attends RC meetings. The Chair of the RC rotates between the teams on a quarterly basis. Joint decisions regarding interventions, data management and handling of biological samples or other technical aspects of the cohorts are taken by the members through a consensus process among the team leads.
HeLTI Coordination and Support
World Health Organization (WHO) Secretariat
The mandate of the WHO Secretariat is to provide scientific and technical support, advice and quality assurance to the various HeLTI governance bodies in order to optimize the collaboration and synergize the scientific activities of HeLTI. The WHO Secretariat also manages potential new partnership opportunities and performs monitoring and evaluation functions relating to quality assurance.
HeLTI Office
The HeLTI Office administratively supports the harmonized approach to data integrity, sharing and interoperability across the HeLTI teams, and works under the direction of the Research Committee.
HeLTI Governance Model
HeLTI International Data Monitoring Committee
The HeLTI Data Monitoring Committee (DMC) was created in 2019. The DMC evaluates the safety, study conduct, scientific validity and data completeness of the studies. Its mandate is to safeguard the interests of study participants and assure the integrity and credibility of the HeLTI trials. The members of the DMC serve in an individual capacity and provide their expertise and recommendations.
The HeLTI DMC Charter was developed jointly with members of the Research Committee and the DMC.
The Charter is available here.
